PeptiStar maintains a company-wide quality assurance system that complies with cGXP requirements to deliver the comprehensive quality required of APIs. As we strive to fulfill our responsibility as the leading Japanese drug discovery company, we will continue our efforts and endeavor to establish improved quality assurance system.
We, PeptiStar globally supply high quality peptide products and oligonucleotide products for human health and a better future of pharmaceutical industry.
Quality Assurance Department
2022/04/01
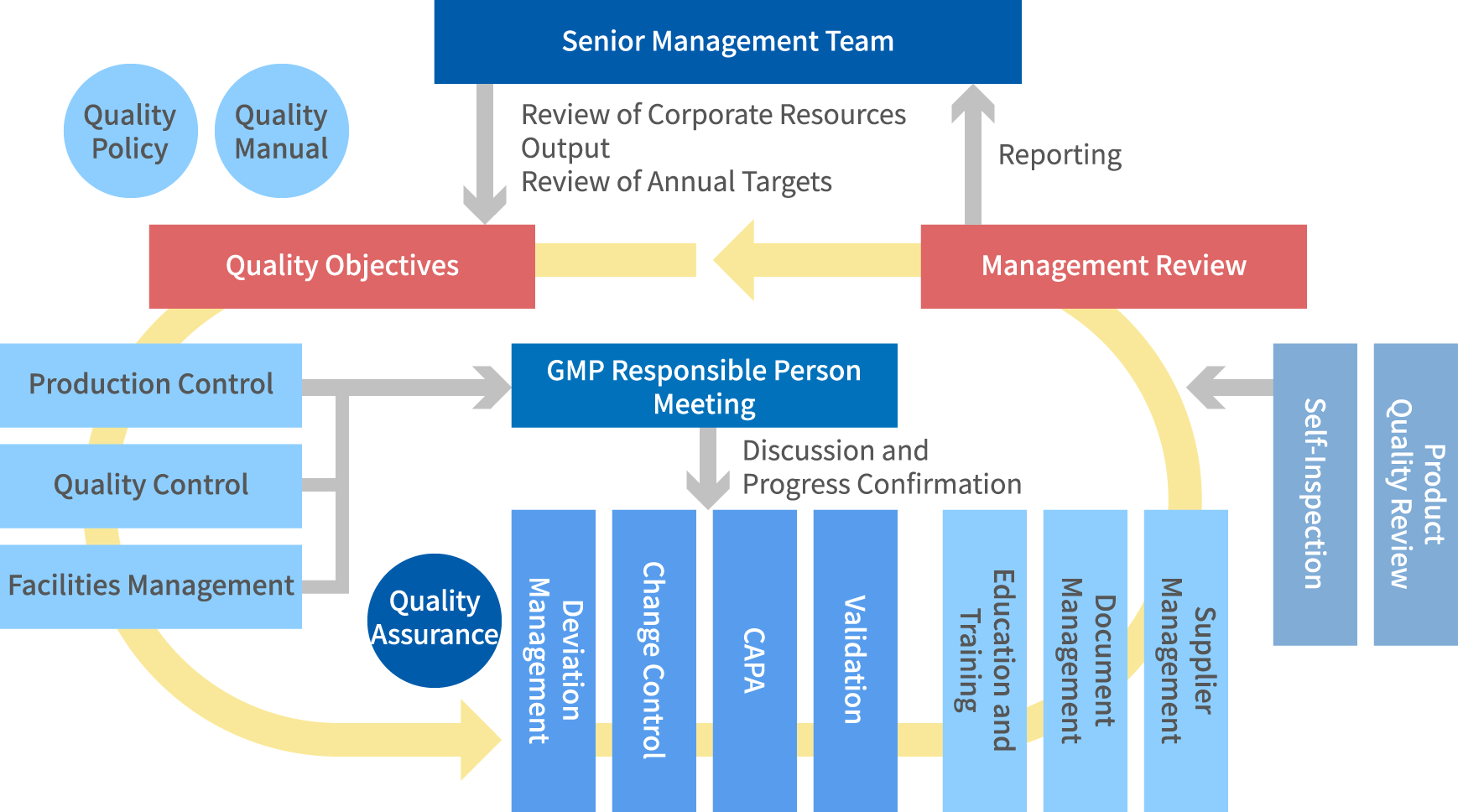
We conduct detailed monitoring of daily activities to ensure operational integrity and accuracy. Meetings of GMP responsible persons, self-inspection and product quality review are performed as appropriate to ensure heightened risk awareness and to prevent problems from occurring. Additionally, the senior management team is open to receiving report from any department at any time, and issues call for action, such as a review of quality objective. The entire product lifecycle is managed at a high level by implementing a company-wide system that encompasses all departments.
PeptiStar guarantees data integrity for all GxP requirements. We comprehensively comply with all ALCOA and CCEA principles from security of various data to the entire facility and operational structure. We continuously maintain and improve the system that assures the reliability of our products. Additionally, we ensure that all staff who work on GxP requirements have the understanding and share the data integrity policy. The practical work is conducted only by the staff who received necessary training.
As a principle, we conduct qualification of all suppliers that are related to GMP processes. Since the quality of raw material directly impacts the product quality, in particular, we evaluate the supplier’s technical competency, confirmation of sample product quality, quality / manufacturing control and situation around observance of product quality assurance system (quality policy, manufacturing control system, quality control system, quality assurance organization etc.), and make comprehensive judgment for each supplier.
