The application of Oligonucleotide pharmaceuticals is expected to satisfy unmet medical needs. PeptiStar started contract services around Oligonucleotides and its conjugates (Peptides + Oligonucleotides) from 2020 by applying technologies and know-hows that were cultivated through Peptide API manufacturing. Our core team of highly experienced staff with superior response capabilities is driving continuous innovation around development and manufacturing of Oligonucleotide APIs.


Synthesis for a wide range of scale is possible, from minimum of several dozen µg to maximum of several Kg. PeptiStar is prepared to propose solutions to meet client needs and intended uses, from labo-scale for research to routine GMP manufacturing, including scale-up evaluation and quality design.

Optimum purification technology can be selected from a variety of methods such as ion exchange chromatography that demonstrates superior selectivity or Continuous Chromatography Purification Method. We have columns up to the maximum of φ450mm. Purification process is conducted under strict sanitary controls in Class 100,000 clean room environment.






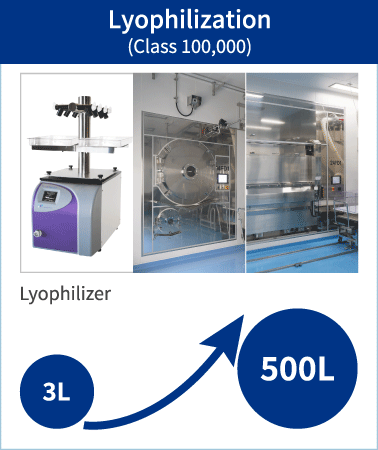
Lyophilization process know-how that we have accumulated in the Peptide API manufacturing can also be effectively applied to Oligonucleotide APIs. We have one of the very few 500L lyophilizer available worldwide and operate the process in Class 100,000 clean room. PeptiStar can deliver high quality and cost competitive APIs.



