PeptiStar can offer solutions in all phases from early drug development to application for approval, including appropriate specifications and testing method for each phase, validation of analytical methods and stability testing. Using a suitable lineup of equipment, our highly skilled staff will conduct analytical method development to quality testing for both Peptides and Oligonucleotides.

Including the LC-MS/MS that are dedicated to Peptides and Oligonucleotides, the structural analysis of both Peptides and Oligonucleotides is done using the following equipment. Our highly experienced staff will provide accurate results of structural analysis.

LC-MS/MS Dedicated to Peptides

LC-MS/MS Dedicated to Oligonucleotides
Samples with sensitive moisture absorption/desorption characteristics are weighed in constant temperature and humidity weighing room. Samples with high toxicity, such as genotoxic impurities, are handled safely inside a balance enclosure.
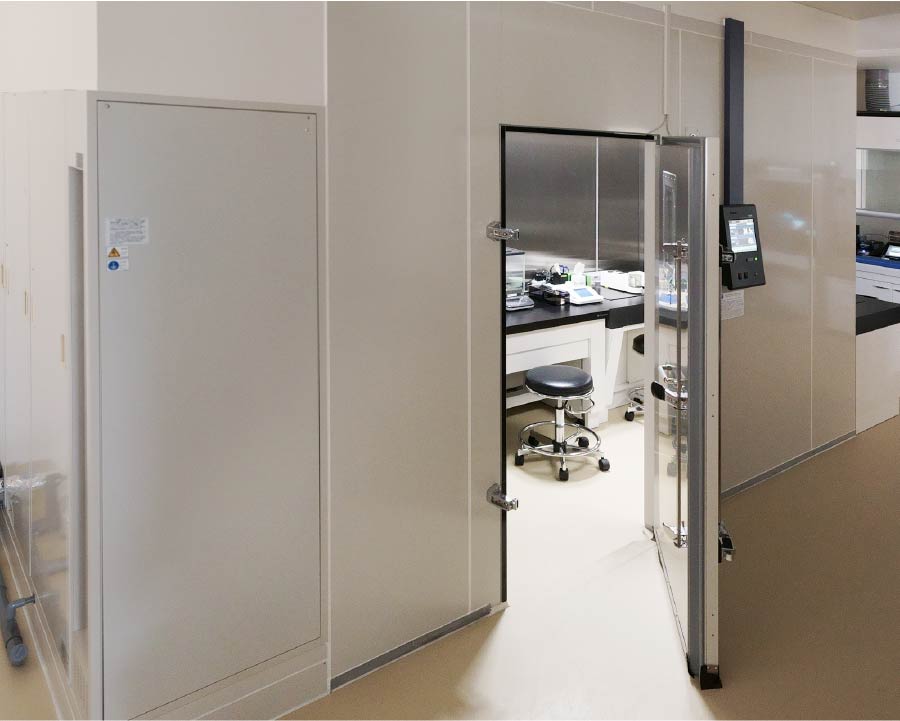
Constant Temperature and Humidity Weighing Room

Balance Enclosure
We conduct stability studies that conform to ICH Guidelines. Following study methods are available, but especially for light exposure test of compound that is unstable to temperature, test can be conducted at 5℃.
