Peptide and Oligonucleotide API Manufacturing Technologies
Since lyophilization process must be repeated depending on the volume of the purification solution, this has a major impact on manufacturing time. It is possible to reduce the purification solution through vacuum concentration, but the difficulty is that Peptides are sensitive to thermal load. On the other hand, membrane concentration that can achieve concentration without thermal load poses challenges around control of the composition, and for Peptides with poor solubility, there are cases where Peptides may precipitate during concentration.
Our New Membrane Concentration Method has overcome these challenges. A new technology was developed that uses FO (Forward osmosis) and MD (Membrane Distillation) from Asahi Kasei Corp., along with a new membrane for this purpose. By concentrating while controlling composition under non-heated / non-pressurized conditions, it is possible to reduce the number of lyophilization processes, achieving shorter manufacturing time.
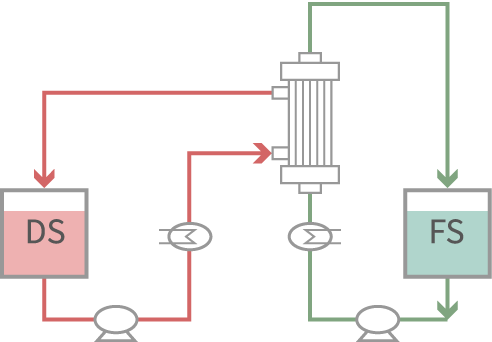
Water is selectively permeated. Water and solvent transfer to the DS (high osmotic pressure) side according to the osmotic pressure difference.
Acetonitrile is selectively permeated. Solvent transfers to the cooling water side according to vapor pressure difference.
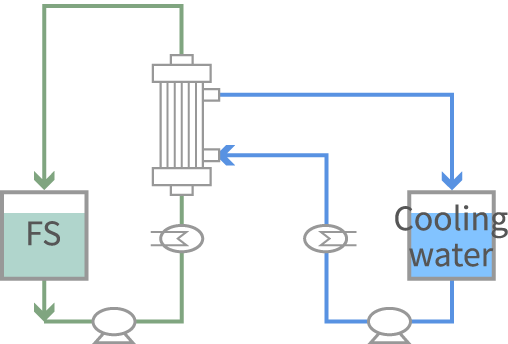
By operating the FO system and the MD system together, composition of the concentration solution can be controlled. Solution to be frozen in the lyophilization process can be obtained under non-heated concentration.
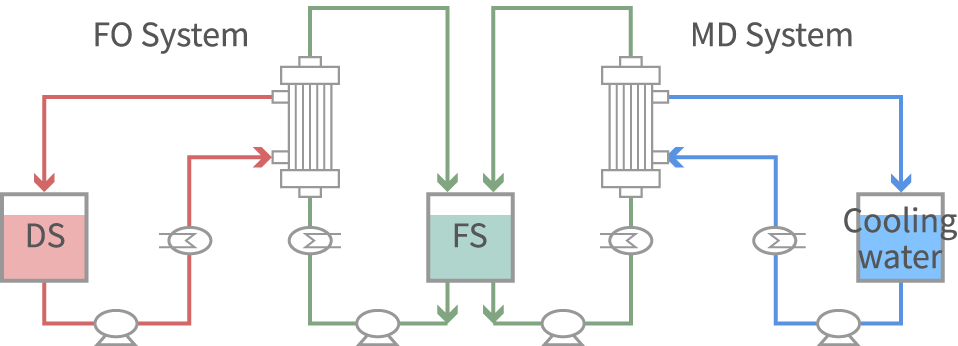
Advantages of New Membrane Concentration Method