Peptide and Oligonucleotide API Manufacturing Technologies
Purification is a critical process that impacts the quality. Generally, yield will decrease if priority is given to API quality, and quality suffers as yield goes up. However, with Continuous Chromatography Purification Method developed by YMC CO. LTD., high quality API can be obtained with high yield. Since the fraction loss is small, synthesis scale can be set small according to target quantity and contribute to significant reduction in raw material cost. This technology is also effective in creating quality design strategy such as improving quality in stages.
PeptiStar is applying the superiority of the Continuous Chromatography Purification Method in manufacturing both Peptide and Oligonucleotide APIs. It can also be used in GMP manufacturing.
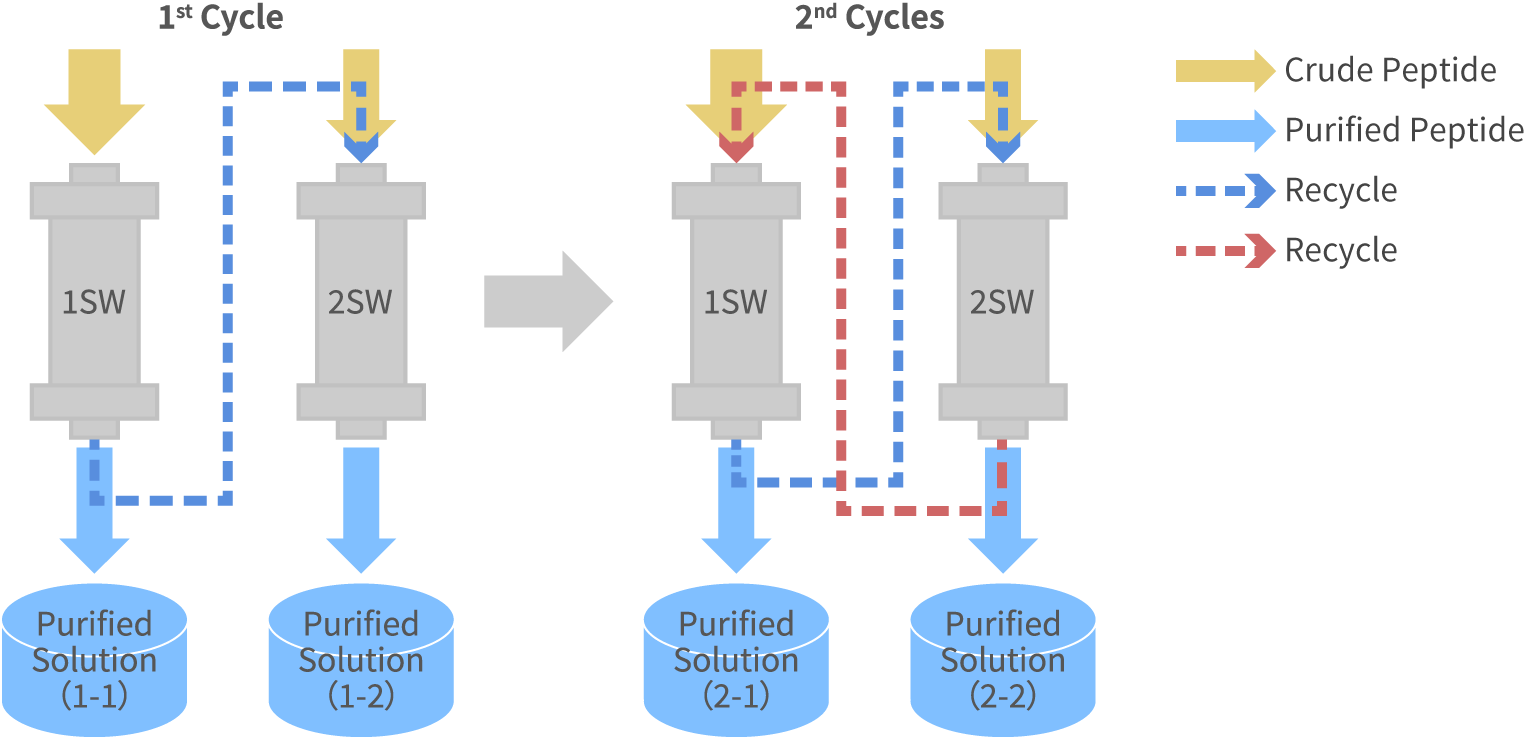
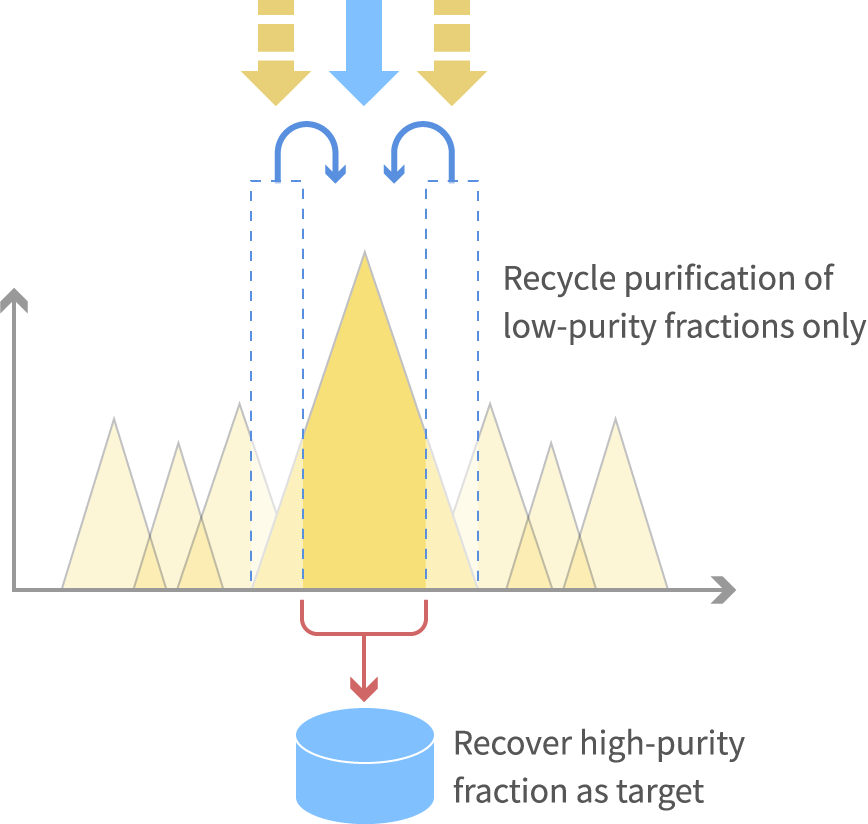
High purity and high yield can be attained by using recycle purification process that combines new sample with low purify fractions that are not disposed.
Advantages of Continuous Chromatography Purification Method
