Development and manufacturing of pharmaceuticals require a wide range of technologies and know-how. PeptiStar leverages the skills of excellent companies throughout Japan to realize a high level of technology competence. As development and manufacturing of Peptide and Oligonucleotide API’s command considerable and growing attention worldwide, PeptiStar brings innovation in this space to offer superb quality and price competitive API’s with short delivery time.
There are two challenges to Peptide manufacturing. The first challenge is low productivity. The Fmoc SPPS method that was developed in the 1970’s is generally the method of choice for Peptide synthesis, but it requires excessive amount of protected amino acids and coupling reagents, resulting in high material cost. The second challenge is a long manufacturing time. All processes, including synthesis, purification and lyophilization, require long time. The long process time translates to processing cost, impacting the product cost which results in lowered cost competitiveness.
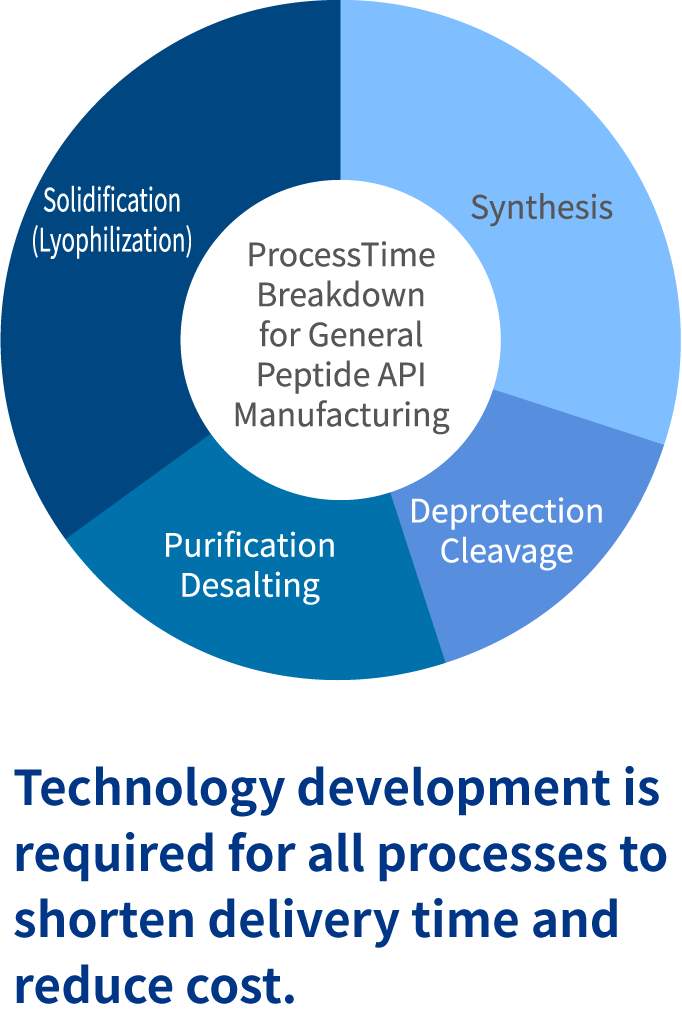
Innovation in technology is indispensable in overcoming the inherent challenges in Peptide manufacturing. For example, if production yield can be enhanced, raw material use can be curtailed, reducing raw material cost. Additionally, shortening the reaction time in synthesis or reducing the volume of lyophilization can lead to shortening the manufacturing time. Building upon our collaboration with innovative Japanese companies, PeptiStar is focusing on the various initiatives around cost reduction and shortening of manufacturing time.
| Synthesis | Purification / Solidification | ||||
| SPPS | LPPS | Purification | Concentration | Lyophilization | |
| Conventional Technology | SPPS Method | LPPS Method | Single Column Reverse Phase | Concentration (Dilution) | Shelf-type Lyophilization |
| PeptiStar Technology | MW SPPS Method | STag-PS Method SYNCSOL® |
Continuous Chromatography Purification Method HILIC |
New Membrane Concentration Method | Mixer-type Lyophilization |
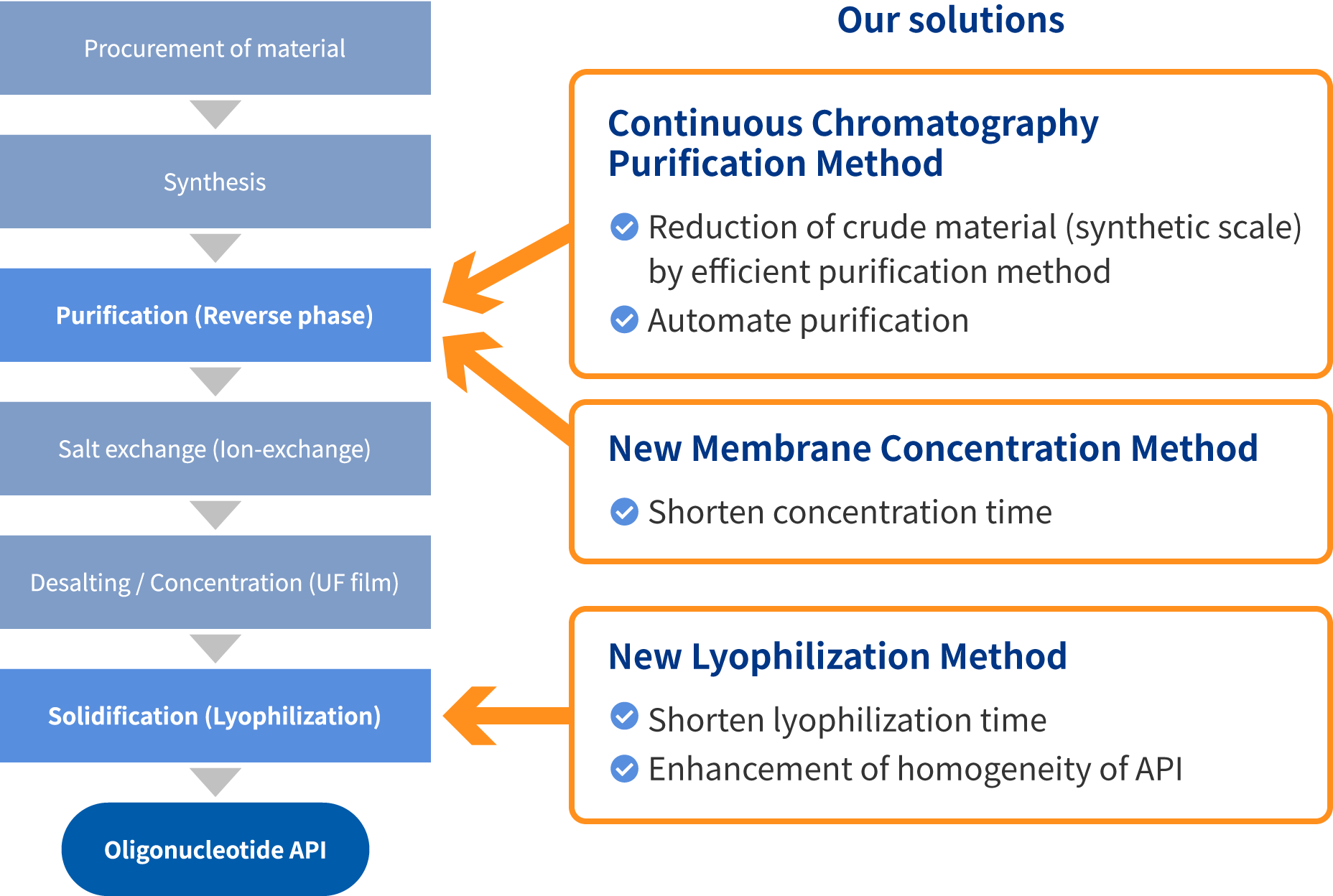
The innovative manufacturing technologies for Peptide API can be applied to manufacturing of Oligonucleotide API. Continuous Chromatography Purification Method to attain high production yield, while maintaining high quality, will greatly contribute to improving the efficiency of manufacturing Oligonucleotide API where there are difficulties around removal of impurities. Additionally, one of our targets is to reduce the cost and time of lyophilization process. In addition to established technologies that we possess, we strive to deliver highly competitive Oligonucleotide APIs by proactively evaluating and applying technologies that are in development.