PeptiStar possesses significant advantages in the Japanese Peptide pharmaceutical field, including manufacturing of special Peptide APIs that are challenging to synthesize. We have state-of-the-art facilities such as the world leading class Microwave SPPS to enable flexibility to cover a wide range of manufacturing scale. We emphasize quality assurance through initiatives like conducting all processes from purification onwards in Class 100,000 environment.

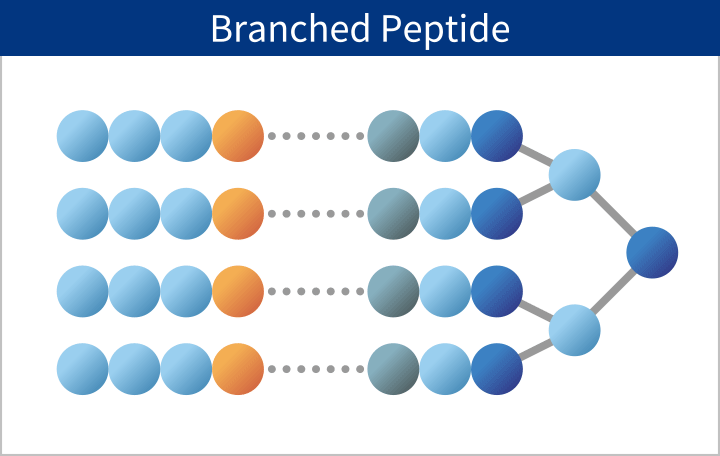
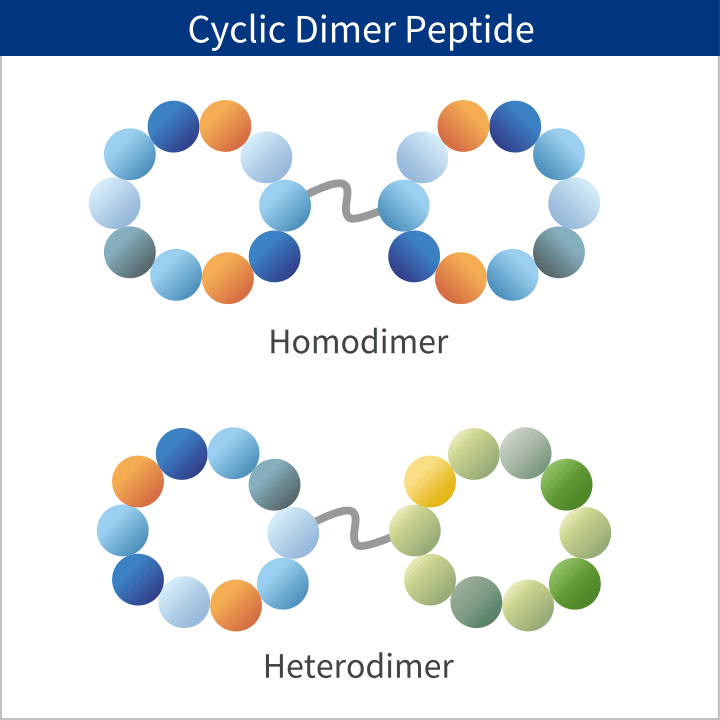
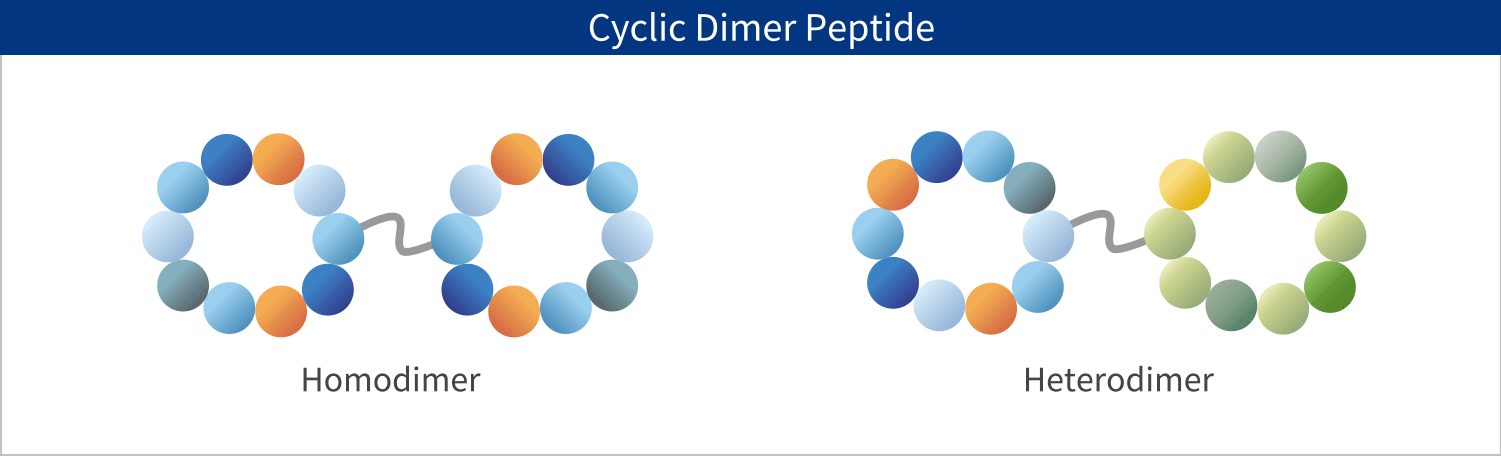

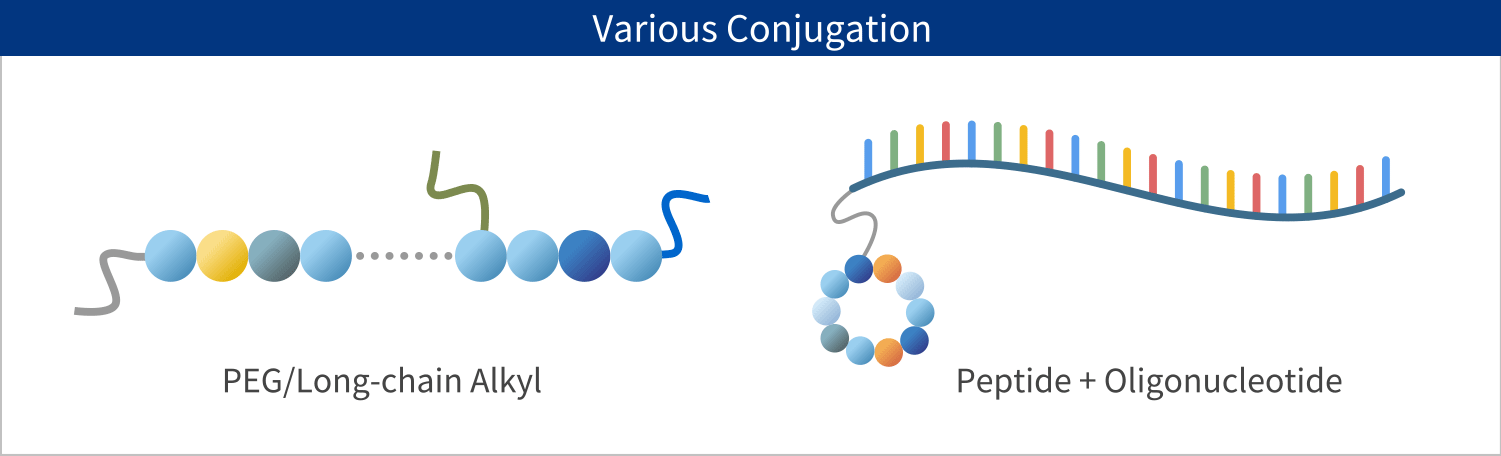
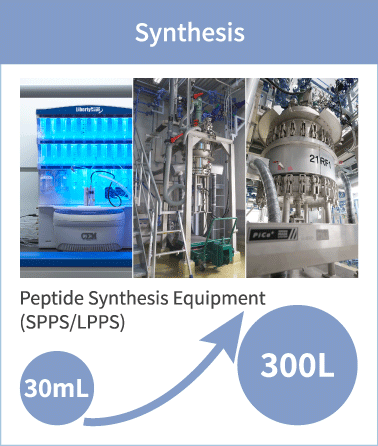

PeptiStar has a 300L Filter Reactor equipment that is one of the largest class in this industry. We can meet wide range of production scale including the commercial production that needs several hundred Kg per year. Additionally, our Microwave SPPS Method that contributes to reduction in process time and energy usage is amenable to scaling up with custom built equipment. LPPS can also be selected as an alternative to SPPS where cost reduction potential can be expected.





Processes from purification onwards are conducted in a clean room that is controlled equivalent of a hospital operating room (Class 100,000). Based on client’s needs, reaction, extraction concentration, filtration, drying and conjugation processes may also be handled in this clean room environment. PeptiStar has columns in the range from φ10mm to largest domestic class φ450mm, enabling us to scale-up to meet client needs.




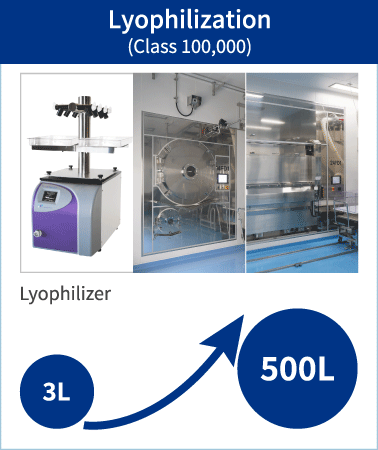
By leveraging a wide scope of knowledge and know how, PeptiStar can develop optimum lyophilization process according to the characteristics of a Peptide. We possess the largest worldwide class of 500L lyophilizer and cleanliness is controlled as Class 100,000. Accurate condition settings and suitable lineup of facilities contribute to producing higher quality product.



