

~Innovative Manufacturing, Advancing Peptide Medicines~

Yasumasa Watanabe (Mr.)
Marketing Responsible,
Vice President,
Corporate Strategy Department
Mail: y-watanabe@peptistar.co.jp
URL: https://peptistar.com
Please feel free to contact us for any questions, inquiries, etc
Tomoaki Masuda (Mr.)
Department Manager,
Fine Chemicals & International Trading
Mail: tmasuda@mitsubishiingredients.com
TEL: +1-973-368-7708
(You can jump to the page from the below hyperlink)



*In case of Solid Phase Peptide Synthesis
** SPPS: Solid Phase Peptide Synthesis, LPPS: Liquid Phase Peptide Synthesis


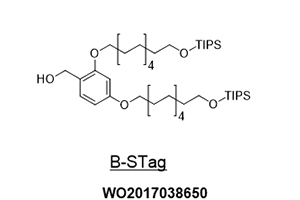
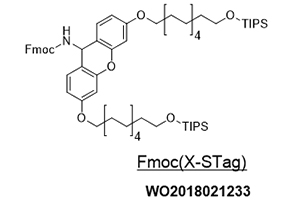

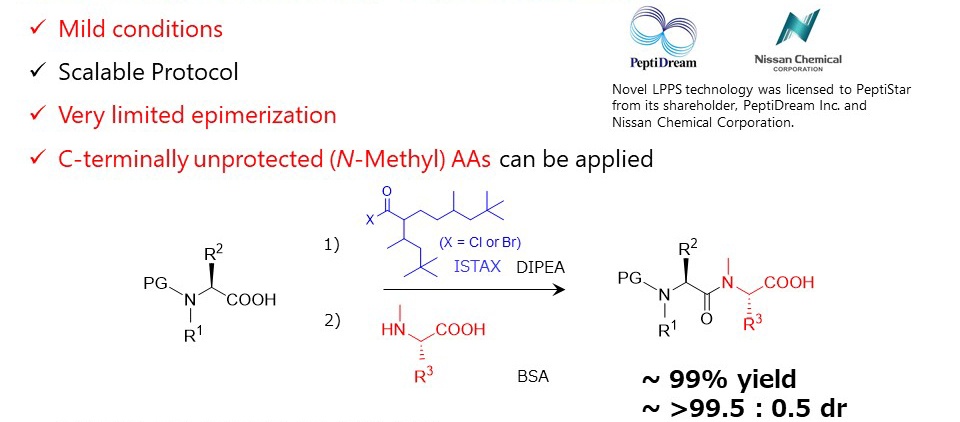
Ref.) Org. Lett. 2020, 22, 20, 8039-8043
https://doi.org/10.1021/acs.orglett.0c02984
WO2020/189621
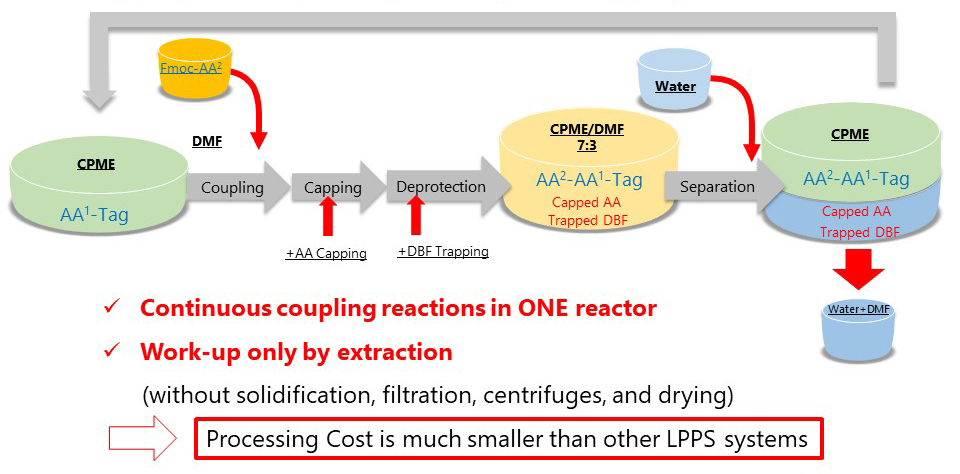
BSA; N,O-bis(trimethylsilyl)acetamide
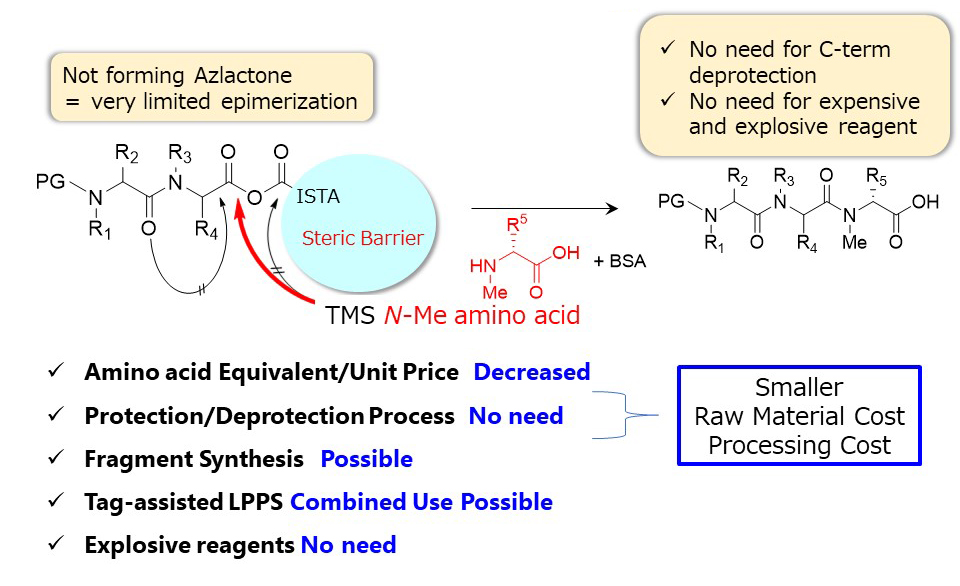
BSA; N,O-bis(trimethylsilyl)acetamide
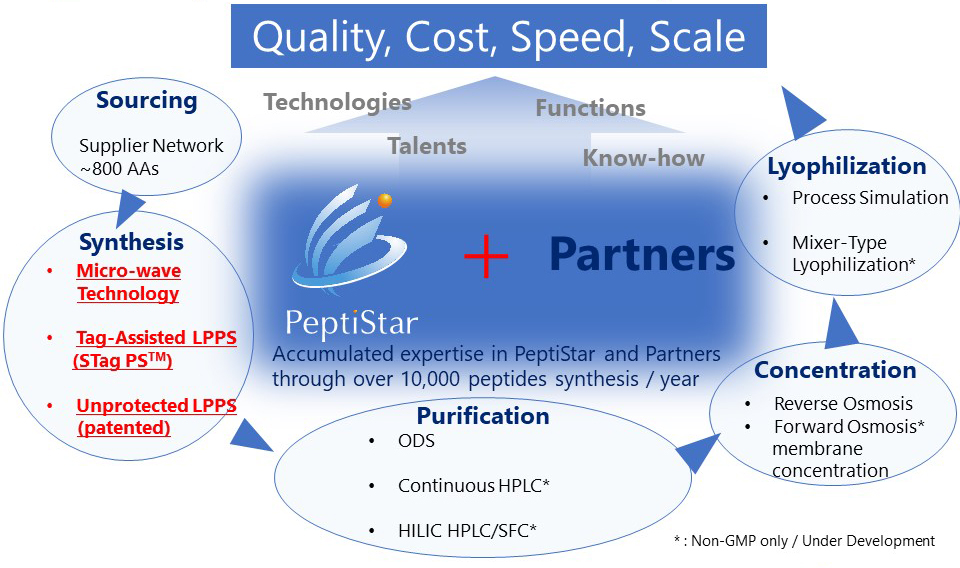
Confidential
|
SPPS |
LPPS |
|||
|---|---|---|---|---|
|
Conventional SPPS |
Microwave SPPS |
Stag-PS |
Unprotected LPPS |
|
| Features |
- |
Quick/Uniform heating |
Serial coupling in CPME |
Using Unprotected AAs |
|
AA equivalent |
2-6 eq. |
1.5-3 eq. |
1.2-2 eq. |
1.1-1.5 eq. |
|
AA unit cost |
high |
high |
high |
High - Low |
|
Rxn. Time |
Long |
Short - Middle |
Short - Middle |
Short - Middle |
|
Monitoring |
Qualitative |
Qualitative |
Quantitative |
Quantitative |
|
Fragment Strategy |
- | - | - | Possible |
|
Filter Reactor |
Necessary |
Necessary |
NOT necessary |
NOT necessary |
|
Scale up |
Limited by facility scale |
Limited by facility scale |
Easy |
Easy |
|
Development time |
Short |
Short |
Middle |
Long |
|
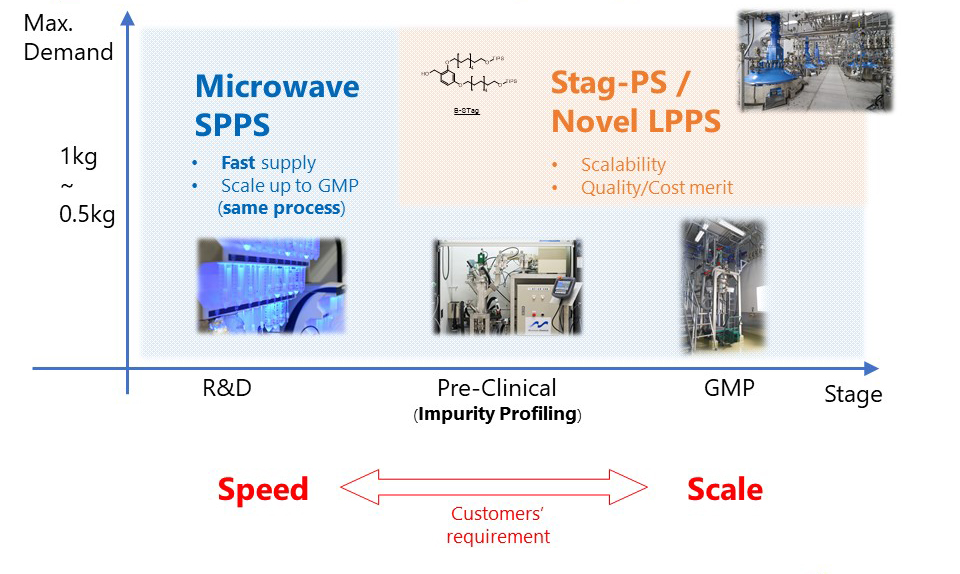
【Suitability to each scale】 |
||||
|
Sample |
★ |
★★★ |
☆ |
☆ |
|
Tox. study |
★ |
★★★ |
★★ |
★★ |
|
Clinical Trial |
★ |
★★ |
★★ |
★★ |
|
Commercial |
★ |
★★ |
★★★ |
★★★ |
Confidential
















|
Process Development |
Manufacturing |
QC/QA/Regulatory |
|
|---|---|---|---|
|
PeptiStar |
|
|
|